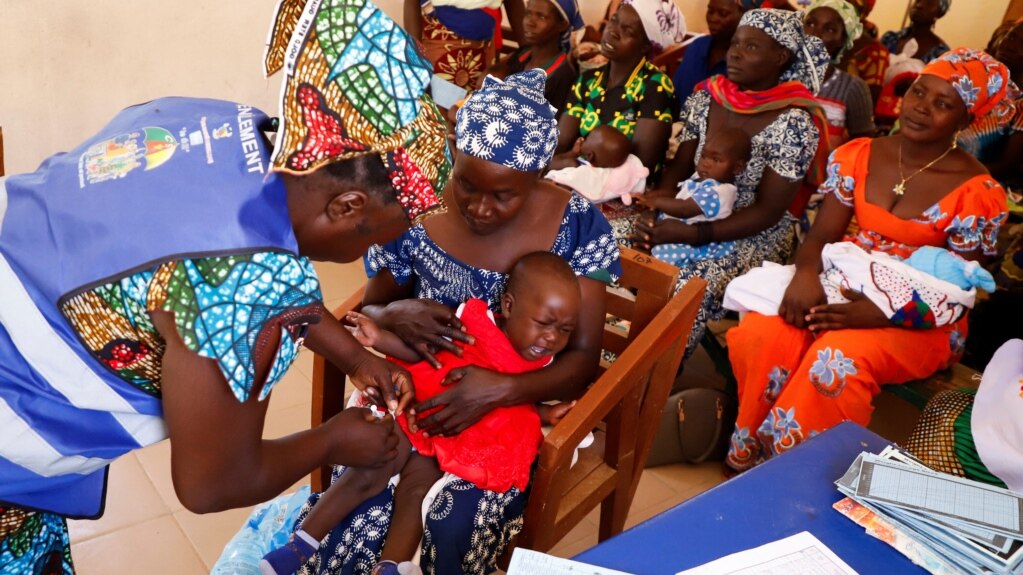
Cameroon has begun the world’s first major malaria vaccine program for children.
The campaign launched Monday in the Central African nation. Health officials called the effort a major step in the fight against the disease across Africa. The continent accounts for about 95 percent of the world’s malaria deaths.
The World Health Organization (WHO) estimates the disease, which is spread through mosquitos, kills more than 600,000 people a year. Most of the deaths involve young children.
The vaccine, called RTS.S, was developed by British drug company GlaxoSmithKline (GSK). It is meant to work with other preventive measures, such as the use of bed nets, to fight the disease.
Cameroon is the first country to offer vaccine injections through a routine program after successful tests, or trials, were carried out in Ghana and Kenya. Cameroon hopes to vaccinate about 250,000 children this year and next.
The international vaccine alliance called Gavi has said 19 other countries aim to launch their own campaigns this year. About 6.6 million children in those countries are to receive malaria vaccinations in 2024 and 2025.
"For a long time, we have been waiting for a day like this," said Mohammed Abdulaziz. He is with the African Centers for Disease Control and Prevention. Abdulaziz spoke during a joint news briefing with the WHO, Gavi and other organizations.
Cameroon is using one of two recently approved malaria vaccines, called Mosquirix. When the WHO approved the vaccine two years ago, officials admitted it was not perfect. But they noted its use could sharply reduce severe infections and hospitalizations.
The GSK-produced shot is only about 30 percent effective. It requires four shots, or doses. Tests have shown the vaccine’s protection begins to weaken after several months. GSK has said it can only produce about 15 million doses of Mosquirix a year.
Some experts believe a second malaria vaccine developed by Oxford University and approved by the WHO in October may be a better solution. That vaccine, called R21, is less costly and only requires three doses.
Launching the second vaccine is expected to result in enough vaccine supply “to meet the high demand and reach millions more children," said Kate O'Brien. She is the WHO's director of vaccinations.
"Having two vaccines for malaria will help to close the huge gap between demand and supply and could save tens of thousands of young lives, especially in Africa," said WHO Director-General Tedros Adhanom Ghebreyesus.
India’s Serum Institute – which helped develop the R21 vaccine –has said it could produce up to 200 million doses of it a year. Gavi has said that vaccine could be launched in May or June.
Some experts have raised questions about the long-term effectiveness of the vaccines. They have questioned whether attention and financing should be drawn away from the wider fight against the disease using established prevention methods.
But health experts at the news conference said the new vaccine launch included community efforts aimed at supporting the campaign. Officials advised individuals on vaccine safety and have urged them to continue to use existing protective methods alongside the vaccines.
I’m Bryan Lynn.